Background: The 2017 European LeukemiaNet (ELN-2017) risk classification system updated AML guidelines in 2022, adjusted FLT3-ITD mutated patients without core binding factor (CBF) or adverse risk markers to intermediate risk groups, according to the clinical trial results of midostaurin on patients with FLT3-ITD not NPM1 mutation and MRD in treatment decisions. However, midostaurin is not approved in mainland china; many patients in China are unable to receive FLT3 inhibitor due to highly cost yet.
Methods: We include newly diagnosed de novo AML patients at our center from January 2017 to December 2021, regardless of the further treatment received. Patients with acute promyelocytic leukemia and AML with other previous myeloid neoplasms were excluded. All patients were diagnosed according to 2016 World Health Organization (WHO) criteria. Clinical data, chromosome and next-generation sequencing (NGS) results, treatment regimen and date of survival were included.
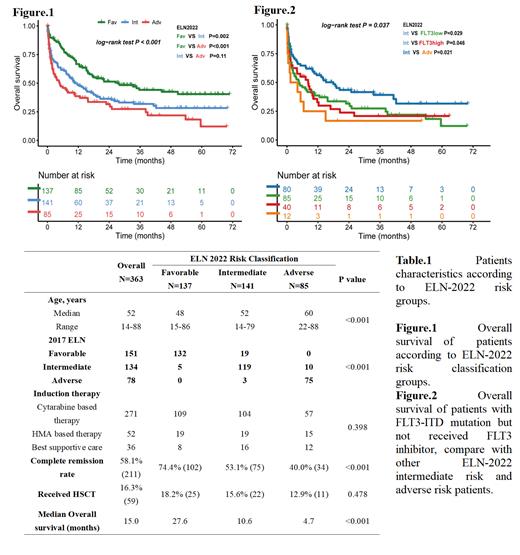
Results: 408 patients were diagnosed as de novo AML (excluding M3) in our center, 364 patients have available cytogenetic and NGS data to access ELN-2022 risk classification. 137 (37.7%), 141 (38.8%) and 83 (23.5%) patients are classified into ELN-2022 favorable, intermediate and adverse risk groups, respectively, the median age of all patients at diagnosis is 52. 37 (10.2%) patients have a change in ELN risk classification compared with ELN-2017. In ELN-2017 favorable risk group, 19 patients whose FLT3-ITD allelic ratio <50% with NPM1 mutation are reclassified to ELN-2022 intermediate risk; in ELN-2017 intermediate risk group, five patients with CEBPA monoallelic bZIP mutation are reclassified to ELN-2022 favorable risk, 10 patients with MR gene mutations are reclassified to adverse risk; in ELN-2017 adverse risk group, 3 patients with FLT3-ITD allelic ratio >50% are reclassified to ELN-2022 intermediate risk. 323 (89.0%) patients received induction therapy: 271 (74.6%) patients received cytarabine-based chemotherapy, 52 (14.3%) patients unfit for intensive chemotherapy received hypomethylating agents (HMA)-based therapy, while 36 (9.9%) patients only received best supportive care, mainly due to early death during hydroxyurea treatment or did not want any therapy due to potential treatment costs. 211 (58.1%) patients received complete remission (CR), with CR rates in the ELN-2022 favorable, intermediate and adverse groups of 74.4%, 53.1% and 40.0%, respectively (p<0.001). After a median follow-up of 36.9 months, the median overall survival (OS) is 27.6 months, 10.6 months and 4.7 months in the ELN-2022 favorable, intermediate and adverse risk groups, compared to 21.9 months, 10.6 months and 4.8 months in the corresponding ELN-2017 risk groups. Patients in the ELN-2022 favorable group have a significantly better OS than those in intermediate and adverse group (p=0.002 and p<0.001), while there are no statistical differences between the intermediate and adverse groups (p=0.11), not significant as ELN-2017 (p=0.046). (Figure.1)
61 patients with FLT3-ITD mutations without adverse prognostic markers are classified as ELN-2022 intermediated risk. Among these patients, only 9 received FLT3 inhibitors (gilteritinib or sorafenib) due to late approval of FLT3 inhibitors in China; the remaining 52 patients received chemotherapy alone: 40 have FLT3-ITD allelic ratio <50%, 12 have allelic ratio ≥50%. These patients unable to receive FLT3 inhibitor did not achieve the same survival outcomes as other ELN-2022 intermediate risk patients (FLT3-ITD allelic ratio <50% group vs. intermediate risk group, p=0.046; FLT3-ITD allelic ratio >50% group vs. intermediate risk group, p=0.021); instead, both FLT3 mutated groups have no survival difference compare to the ELN-2022 adverse risk group. Moreover, for the ELN-2022 intermediate risk group without FLT3-ITD mutated patients, we find statistical difference to ELN-2022 adverse risk group on overall survival (median OS 17.6 months vs. 4.6 months, p=0.029), which is not achieved when FLT3-ITD mutated patients is included. (Figure.2)
Conclusion: our real-world clinical data show that the ELN-2022 risk classification system is not superior to the ELN-2017 version in classifying patients in our center. For patients with FLT3-ITD mutation but unable to receive FLT3 inhibitor, their risk classification and treatment therapy should be considered as adverse risk group.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal